INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most frequent leukemia, being more frequent in older adults.
Bruton's tyrosinkinase inhibitors (BTKi) have revolutionized the treatment, displacing immunochemotherapy treatment in all patient profiles.
The selectivity profile in the action of these drugs means that the toxicity of first and second generation BTKi is different, resulting in higher rates of atrial fibrillation and arterial hypertension, mainly in patients treated in the second line or later, while maintaining their efficacy.
Real-life studies are needed to be able to demonstrate the effects in first-line treatment in a more heterogeneous patient profile, without the strict exclusion and inclusion criteria used in clinical trials.
METHODS
An observational, retrospective, multicenter, retrospective study was performed. Patients with in treatment with Ibrutinib or Acalabrutinib in first line or later were included. Demographic data, comorbidity, cytogenetic and mutational disease data, adverse effect profile, comprehensive geriatric assessment (if applicable) and treatment response evaluation data are collected.
RESULTS
A total of 91 patients were included with a median age of 73.22 years SD 11.06. Fifty-three (58.2%) were male. Fifty-three (58.2%) were treated in frontline, 31.9% (29) in second line and 9.9% (9) in subsequent lines.
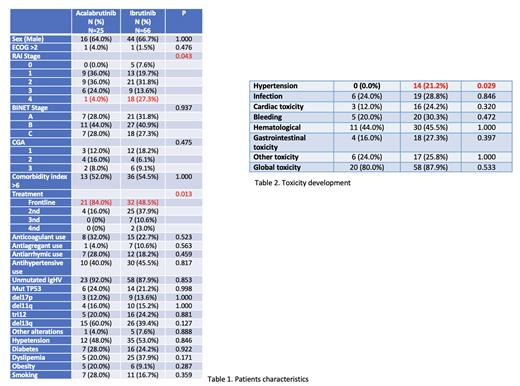
Acalabrutinib was used in 25 (27.5%) patients and Ibrutinib in 66 (72.5%). Patient characteristics are shown in Table 1.
Differences were revealed between these two BTKis in terms of line of treatment, with the majority being treated first (P=0.006) and Rai stage, with Rai II predominating (P=0.014). No differences were found between the two BTKis according to the rest of the characteristics.
In terms of toxicity, 14 (15.4%) of patients treated with Ibrutinib developed arterial hypertension, compared to no patients in the Acalabrutinib arm (p=0.009).
With respect to the development of cardiac toxicity, 19 (20.9%) patients suffered some cardiotoxic event, 3 (3.3%) in patients on acalabrutinb and 16 (17.6%) on ibrutinib, with no significant differences found between the two drugs (p=0.2). No significant differences were found with respect to other toxicities (hematologic, digestive, bleeding and infections). Table 2
No differences were found in terms of progression-free survival or overall survival.
CONCLUSIONS
The iBTKs have changed the treatment of CLL, although the second-generation iBTKs have a safer cardiovascular profile and are just as effective in our cohort, even in the frontline.
Disclosures
Lopez Garcia:Janssen: Consultancy, Speakers Bureau; Beigene: Consultancy; Roche: Consultancy, Speakers Bureau; Astrazeneca: Consultancy, Speakers Bureau. Morillo:ABBVIE: Honoraria; GSK: Honoraria. Cordoba:F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Consultancy; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Speakers Bureau; European Hematology Association (EHA), Spanish Society Hematology (SEHH): Membership on an entity's Board of Directors or advisory committees; Fundacion Jimenez Diaz University Hospital: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal